‘Q’ is for Quality. In the context of a management system, when the dimension of quality is incorporated into the management model, everything is possible. At a minimum, expectations are met, important efficiencies are realised, and the system is embraced as an improvement to how things were previously done. But what do these quality processes look like? The framework for a Quality Management System (QMS) was established by international standards, ISO 9001 Quality Management System – requirements. The standard was first published in 1994 and has since been republished in 2000, 2008, and the latest in 2015.
Based on the ISO 9001 model, the core element of a quality management system is that it supports the PDCA cycle – plan, do, check, act. It sounds onerous, but we use the methodology intuitively in most things we do every day. Take dropping the kids off at school, for example. Immediately you calculate when you need to leave home to get there on time and the route you will take (Plan). And when you are on the road, you’re constantly calculating time and distance (Do, Check) and if you’re late, you’ll take the learnings on and do it differently next time (Act).
The benefit of using a technology-based QMS is that the interrelationship between these processes of planning, doing, checking, and acting can, and should, be seamless.
Compliance and Continuous Improvement
Managing quality in an organisation requires effectively managing a range of processes within and across teams. For example, risk management, safety management, contract management, people management, and program/service management. The QMS therefore needs to have ‘controls’ over processes to ensure what needs to be done is done, to a specified standard, and within a required timeframe. These ‘controls’ include:
- Clear delegations for responsibility and accountability for task management;
- Clear instructions for completing tasks and;
- Built-in checking processes to ensure what has been done meets requirements.
But a commitment to quality is more than just managing compliance (Plan, Do, Check). It also requires a commitment to continuous improvement (Act). There are two strategies to identify opportunities for improvement: proactively through an internal audit program, and reactively through using the learnings arising from adverse events. Both strategies require support from a QMS.
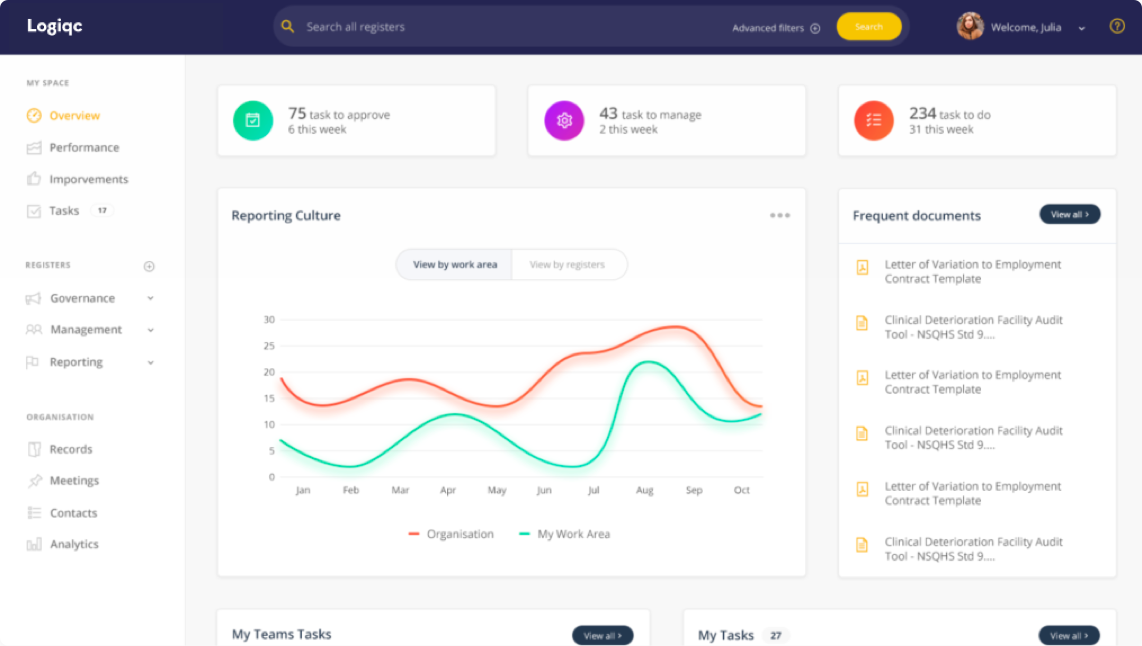
The importance of a strong reporting culture
The more difficult of the two relies on a strong reporting culture, both within the organisation and the people who they serve. To support this culture, the QMS needs to have the functionality that:
- Enables real-time reporting of adverse events;
- Supports managers to be responsive, through automated delegations;
- Keeps individuals and teams informed of outcomes;
- Tracks progress and status in managing adverse events;
- Provides trend reporting from a range of perspectives, including process, service area and advent type;
- Prompts consideration of improvements.
When teams understand the benefits of reporting and know that as a result, issues will be addressed and improvements will be made, organisations have set a foundation for a strong reporting culture. While this cannot be achieved by a QMS alone, the system needs to have the bells and whistles that enable a reporting culture.
The distinction and benefit of a Quality Management System from a Management System is its purpose. The ultimate purpose of a QMS is to support an organisation to become a “learning organisation”. To achieve this, the QMS needs to have the scope and functionality to provide a deep understanding of where system and process improvements are likely to make the greatest gains. Thereby enabling the organisation to become better at what it does and what it provides.
If you’re ready to put the ‘Q’ into your QMS, get in touch to learn more about whether LogiqcQMS is the right fit for your organisation.