Save It, Finish It, Submit It: How “Save as Draft” Brings Flexibility to Your Workflow
Ever started a report or audit only to be interrupted halfway through? With Logiqc’s new Save as Draft feature, that’s no longer a problem.
Product Update : Improved system menu management - Learn More
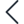
Implementing a comprehensive QMS platform can feel complex. Logiqc simplifies the transition with dedicated onboarding and a suite of services designed for rapid deployment.
We're with you every step of the way. In addition to onboarding, we provide comprehensive support services, from importing your existing data (risks, documents, and assets) to offering a sandbox environment for testing and training, regular platform health checks, and expert guidance on using the platform's full functionality.
Migrating your documents is easy with Logiqc. We'll guide you through the process, from data architecture planning to automated import. Our service supports most formats and includes an import tool. The standard package covers up to 1000 documents. Need help with a larger migration or prefer a full-service approach? We can tailor a solution to fit your needs – just get in touch.
Let us help you build your Asset Register. We provide expert support, from data architecture planning to importing your approved assets. Two imports are included over six months.
We'll help you build your Risk Register. Our support includes data architecture planning and a tool to import your draft risks.
A solid foundation for your Risk Register. We provide a pre-built register with approximately 50 typical risks for health and community service organisations, giving you a strong starting point that you can adapt to your specific needs.
Take control of your testing and training with a dedicated sandbox environment, complete with five pre-configured users and flexible licensing.
Understand the effectiveness of your QMS with two detailed Health Check Reports. The initial report establishes baseline performance, and the follow-up report provides a comparative analysis and recommendations for continuous improvement.
Get the most out of your Logiqc platform with our Optimization Advisory. Two one-hour sessions with a Logiqc expert will provide best-practice insights and tailored advice specific to your organisation's needs and objectives. We can help with platform optimisation, data architecture, and workflow design.
Simplify ISO 9001:2015 compliance with Logiqc's documented procedures. These resources provide a complete overview of the platform's functionality, are ideal for training, and significantly reduce the burden of preparing documentation for external audits.
The procedure aligns with ISO 9001:2015 and ISO 31000:2018 Risk management – Guidelines. It defines the organisation's ongoing process of taking actions (quality improvement) to address risks and opportunities and covers reporting and implementing the improvements in the Logiqc.
The procedure aligns with ISO 9001:2015. The scope of the Supplier Guidelines includes those suppliers who your organisation may purchase goods and services from that may have the potential to affect the quality of your services and therefore have a bearing, direct or indirect, on customer outcomes.
The Clinical Review Committee (CRC) Terms of Reference (TOR) have been developed to align with the requirements of the ISO 9001 Management Review. The TOR for the CRC positions the CRC as a committee with overall responsibility to review and evaluate the performance of clinical operations and programs and services and to make recommendations to the Management Review Committee. Includes an example Meeting Agenda and Minute Template.
This procedure aligns with ISO 9001:2015 requirements. Its intent is to describe how the Logiqc Feedback register manages feedback from external stakeholders, in terms of how access to feedback can be controlled, how feedback will be managed by the in-built workflow in the platform, and the link between the feedback and improvement registers.
This procedure aligns with ISO 9001:2015 requirements. This policy describes the process for the creation, approval, revision, archiving and disposition of controlled documents and records within the organisation’s Logiqc quality management system.
This procedure aligns with ISO 9001:2015 requirements. It describes the process for the control and disposition of non-conforming products, processes, services, equipment and materials. Non-conforming product can mean any process, service or product that is considered not to conform with agreed specifications, policies, and procedures.
This procedure aligns with ISO 9001:2015 requirements. It defines the requirements for reporting and managing incidents in the Logiqc platform. The procedure steps through how access to reported events can be controlled; how the incident will be managed as it moves through the built-in workflow; and the linkage between incidents and risks.
This procedure aligns with ISO 9001:2015 requirements and steps through the requirements for determining the internal audit program, procedures for the selection of and responsibilities of internal auditors, confidentiality requirements of auditors, and the procedures for scheduling, conducting, and approving audits. It also refers to the linkage between audits and opportunities for improvements.
These Terms of Reference have been developed to align with ISO 9001:2015 requirements. The Terms of Reference for the Management Review Committee (MRC) positions the MRC with overall responsibility for the review and evaluation of the performance of the organisation. Includes an example Meeting Agenda and Minute Template.
This procedure aligns with ISO 9001:2015 requirements and defines the organisation's ongoing process of quality improvement and covers management of nonconformities and opportunities for improvement.
Explore the stories below to hear how Logiqc enhances safety, quality, and risk management.

"We recently opened a new dental clinic and it was accredited within 4 weeks; we would normally have given ourselves up to a year to get it done."
Leonie Smit - Risk, Compliance & Quality Manager at Institute for Urban Indigenous Health

"Audits and accreditation can be a stressful experience, having extra support from a team of people who do this for a living is incredibly valuable."
Darcy Inglis - Managing Director at Honestally

"We received great feedback from the assessor that he hadn’t seen a QMS like Logiqc being used to its full capacity and working so well."
Cassie Atchison - CEO at Broome Regional Aboriginal Medical Service (BRAMS)

"We use LogiqcQMS predominantly for ISO 9001 accreditation. I don’t think we would have achieved the accreditation if we didn’t have it."
Claire Wimbridge - Quality & Compliance Manager at East Kimberley Job Pathways

"Having confidence in the system, allowing access to all staff, and knowing that the information is accurate and current is important to us."
Sally Sibley - Quality Manager at Ramahyuck District Aboriginal Corporation

"We are up to about 230 reporting compliances and they are all loaded into the platform.[...] LogiqcQMS keeps everyone accountable."
Floyd Leedie - Chief Executive Officer at Goondir Health Services

"I love it when someone puts an incident up because it won’t get lost until it’s dealt with. It’s a protection for the business financially too."
Bonnie Buchan - Infection Control and Health and Safety at Auckland City Surgical Services

"We added the Risk module about a year ago and review these on a regular basis to decide if any changes are needed. All our incidents then go into LogiqcQMS"
Kim Passante - Clinic and Quality Manager at Carbal Medical Services

"LogiqcQMS definitely helps us to better manage our incidents and feedback. It gives us greater oversight into what is really happening within the business."
Deb Boyd - CEO at Auckland Eye

"LogiqcQMS encourages staff to start analysing risk in the early days of operations."
Leith McMillan - CEO at Day Hospital Consulting
This is an example paragraph where you can talk about whatever you want. This is an example paragraph where you can talk about something.
All Rights Reserved © Logiqc - ABN 79 120 710 769
Privacy Policy